Indications
BETASERON® (interferon beta-1b) is a prescription medicine used to treat relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
BETASERON in Newly Emerging Multiple Sclerosis For Initial Treatment (BENEFIT)

Primary endpoint
BETASERON Efficacy in the BENEFIT Study
2-year study:
5-year study:
Probability of CDMS at Years 2 and 51,2,*
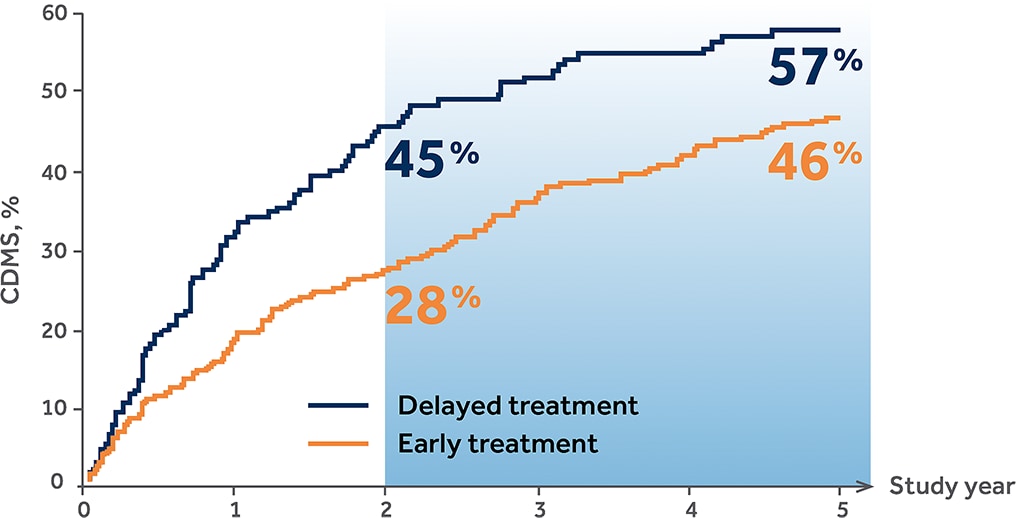
*By proportional hazards regression adjusted for age/gender/steroids/T2 lesions/Gd+ lesions.
Safety Results in the BENEFIT Study
Most common AEs, ≥10%4

To reduce the potential for flu-like symptoms in both studies, mitigation strategies were used, such as dose titration and/or premedication with NSAIDs.1,4
¶Integrated analysis of 2- and 5-year studies.
Incidence of SAEs* and Discontinuation Rates
2-year study:
5-year study:
Discontinuations
2-year study:
5-year study:
AE, adverse event; CDMS, clinically definite multiple sclerosis; CIS, clinically isolated syndrome; Gd+, gadolinium enhancing; MRI, magnetic resonance imaging; MS, multiple sclerosis; NSAID, nonsteroidal anti-inflammatory drug; SAE, serious adverse event; SGOT, aspartate aminotransferase (AST); SGPT, alanine aminotransferase (ALT).
*SAEs were classified as any untoward medical occurrence that at any dose results in death, is life-threatening, requires inpatient hospitalization or prolongation of existing hospitalization, results in persistent or significant disability/incapacity, or is a congenital anomaly/birth defect.4
References: 1. Kappos L, Polman CH, Freedman MS, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology. 2006;67(7):1242-1249. 2. Kappos L, Freedman MS, Polman CH, et al. Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol. 2009;8(11):987-997. 3. Kappos L, Freedman MS, Polman CH, et al. Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3-year follow-up analysis of the BENEFIT study. Lancet. 2007;370(9585):389-397. 4. Data on file. BENEFIT study summary. Bayer HealthCare Pharmaceuticals Inc. Whippany, NJ. 5. BETASERON [prescribing information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc.; Rev. August 2019.



